[ad_1]
Nobel Prize in Physics 2023: French physicists Anne L’Huillier and Pierre Agostini, and Hungarian-Austrian physicist Ferenc Krausz were awarded the 2023 Nobel Prize in Physics on October 3, 2023. They received the Physics Nobel for creating flashes of light that are short enough to capture the extremely rapid movements of electrons. L’Huillier is the fifth woman to win a Physics Nobel.
According to the Nobel Prize organisation, they were awarded the Nobel “for experimental methods that generate attosecond pulses of light for the study of electron dynamics in matter”.
An attosecond is a unit of time and is equal to 10^(-18) of a second. Pulses are flashes of light. By generating attosecond pulses of light, or flashes of light that have an extremely small duration, one can study the movements of electrons.
L’Huillier made infrared laser light interact with a noble gas, as a result of which electrons emitted energy in the form of ultraviolet light. The wavelengths of the ultraviolet light emitted by the electrons were in attoseconds.
Agostini and Krausz leveraged the effect discovered by L’Huillier to generate attosecond pulses, measure them, and also to isolate individual attosecond pulses. The pulses of light created with the help of the effect discovered by L’Huillier were shorter than the pulses of light created using previous technologies.
Such attosecond pulses can be used to measure the rapid movements of electrons because these are light pulses of very short duration, and a short exposure period is necessary to observe something which moves at a fast pace.
Before delving deep into the experiments conducted by L’Huillier, Agostini, and Krausz, let us understand how the field of attosecond physics came into being.
From femtoseconds to attoseconds
Have you ever wondered why humans hear a whirring sound and see a blurred movement when a tiny hummingbird beats its wings? This is because a hummingbird beats its wings 80 times per second, and human senses cannot perceive extremely short events.
In order to capture phenomena which occur in short durations, high-speed photography and strobe lighting, in which a device produces regular flashes of light, are used because these techniques allow the picture to be taken in a shorter exposure time.
We can compare the movements of electrons in an atom to the movement of a hummingbird’s wings. Just as high-speed photography is needed to capture images of the wing’s movements, attosecond pulses are required to observe the movement of electrons.
Inside a molecule, atoms move and turn in millionths of a billionth of a second, or femtoseconds. A femtosecond is equal to 10^(-15) of a second. One can study movements lasting femtoseconds with the help of short pulses produced with a laser.
However, electrons move much faster than atoms. They move and turn in an atom in one billionth of a billionth of a second, or an attosecond.
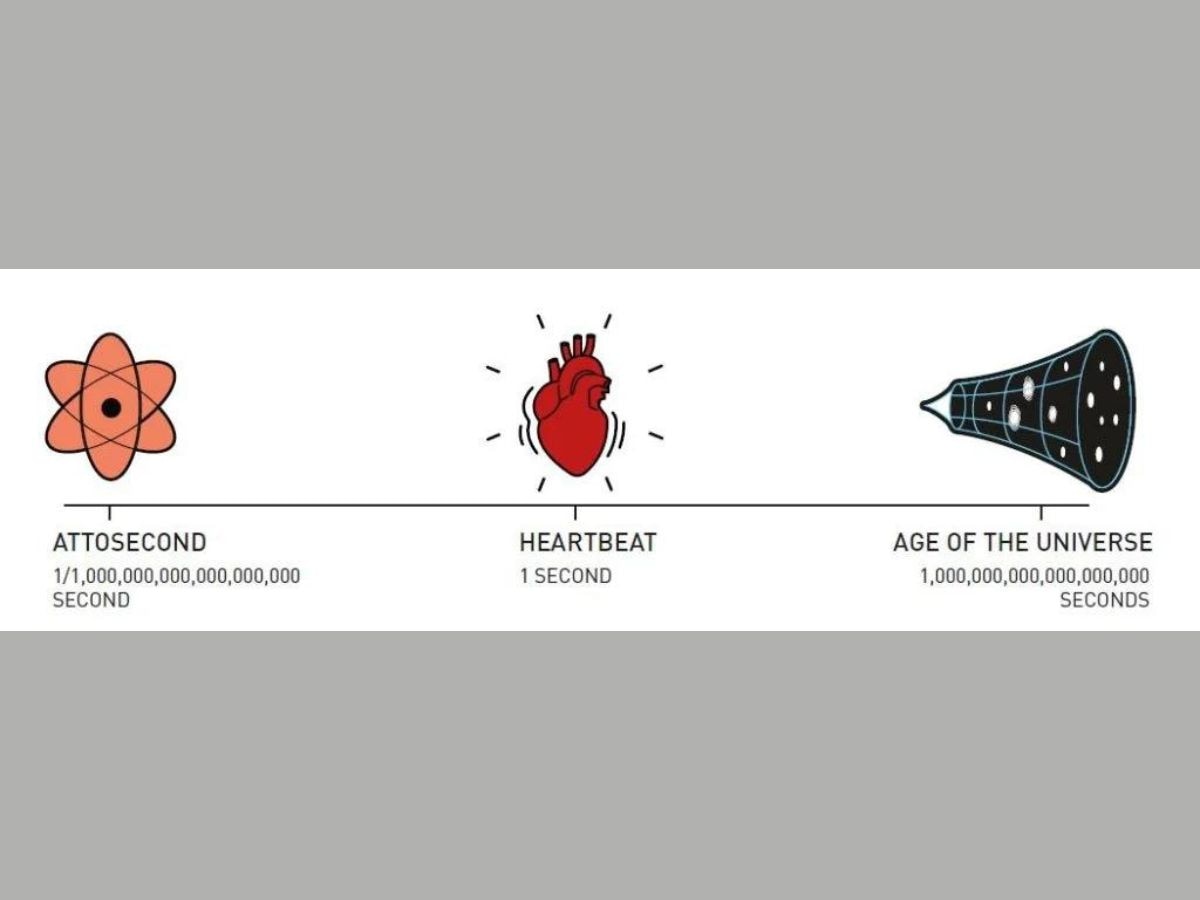
Attoseconds can be understood better by relating this unit of time with the duration of a heartbeat, and the age of the universe.
There are 10^18 attoseconds in a second. One heartbeat lasts one second. The age of the universe is 10^18 seconds. Therefore, the number of attoseconds in a second is equal to the number of seconds that have elapsed since the universe or born. One can also say that the ratio of an attosecond to the duration of a heartbeat is equal to the ratio of a heartbeat to the age of the universe.
The types of movements that can be captured with the help of pulses of light lasting femtoseconds are limited. It is the experiments of this year’s Nobel laureates in physics that have introduced us to the world of attosecond physics.
Now a question arises about how the physicists created short pulses of light. In order to understand this, it is important to know what the shortest possible pulse of light means.
Generating short pulses of light
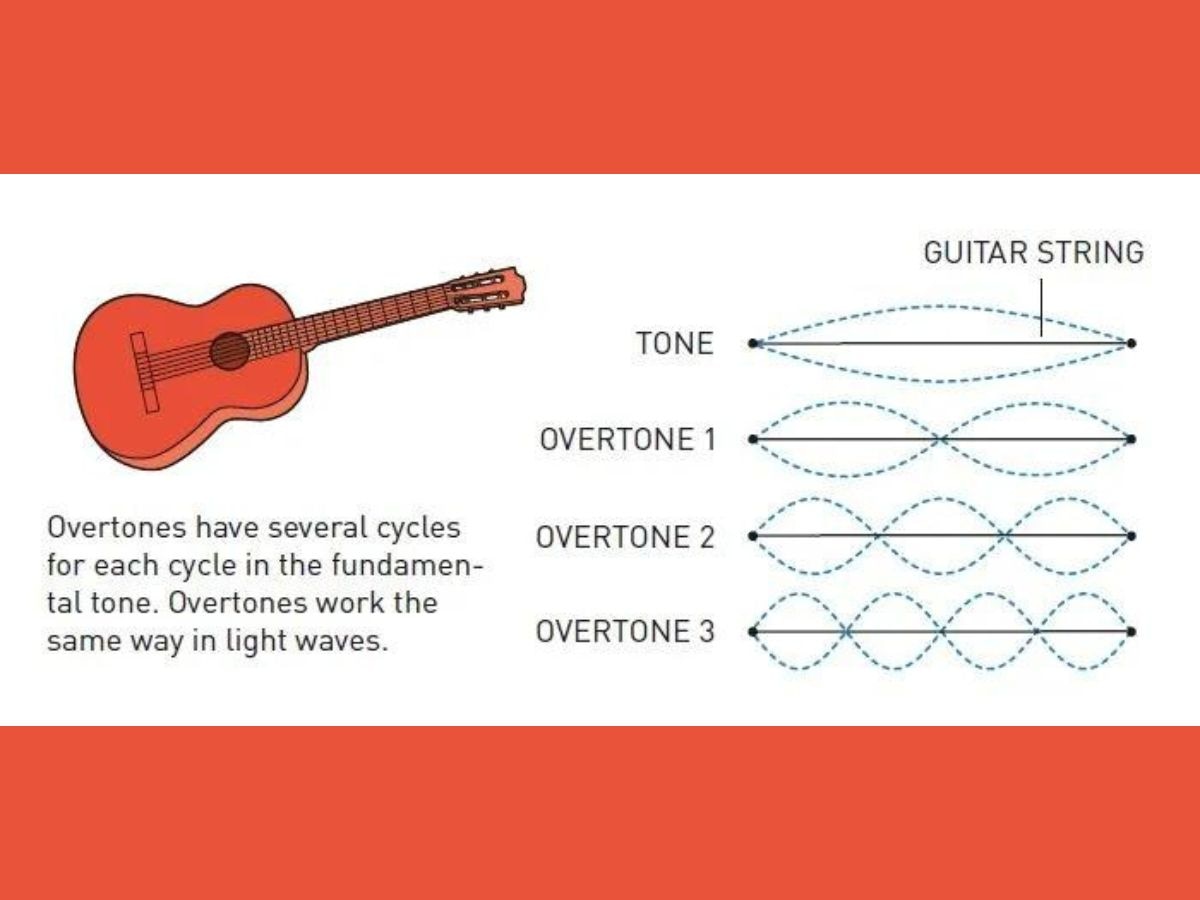
The length of a single period in a light wave is the shortest possible pulse of light. A single period in a light wave refers to the portion of the cycle from the starting point to the peak to the trough and back to the starting point.
Till the 1980s, the shortest possible pulses of light had a duration of a femtosecond.
If enough waves of the appropriate sizes, wavelengths and amplitude are used, one can create waves of any form. If several shorter wavelengths are combined, attosecond pulses can be created.
This is possible by passing laser light through a gas. It allows one to access the briefest instant ever studied: an attosecond.
When laser light interacts with the atoms of the gas, overtones are produced.
Overtones are frequencies greater than the fundamental frequency, which refers to the lowest frequency produced by any instrument. In overtones, there are several cycles for each cycle in the fundamental tone, according to the Nobel Prize organisation.
After the overtones are produced, they must be combined in such a way that concentrated attosecond pulses are produced. When laser light interacts with gaseous atoms, the electromagnetic vibrations produced distort the electric field holding the electrons close to the nucleus. It is due to this electric field that electrons are not able to leave the atom.
However, when the laser pulse distorts this atomic field, the electron escapes the field, and gains lots of extra energy from the electrical field of the laser light.
Since the laser’s electrical field continuously vibrates and keeps changing its direction, the loose electron is pulled back towards the atom it escaped from. In order to reattach itself to the nucleus, the electron must release its excess energy as a pulse of light.
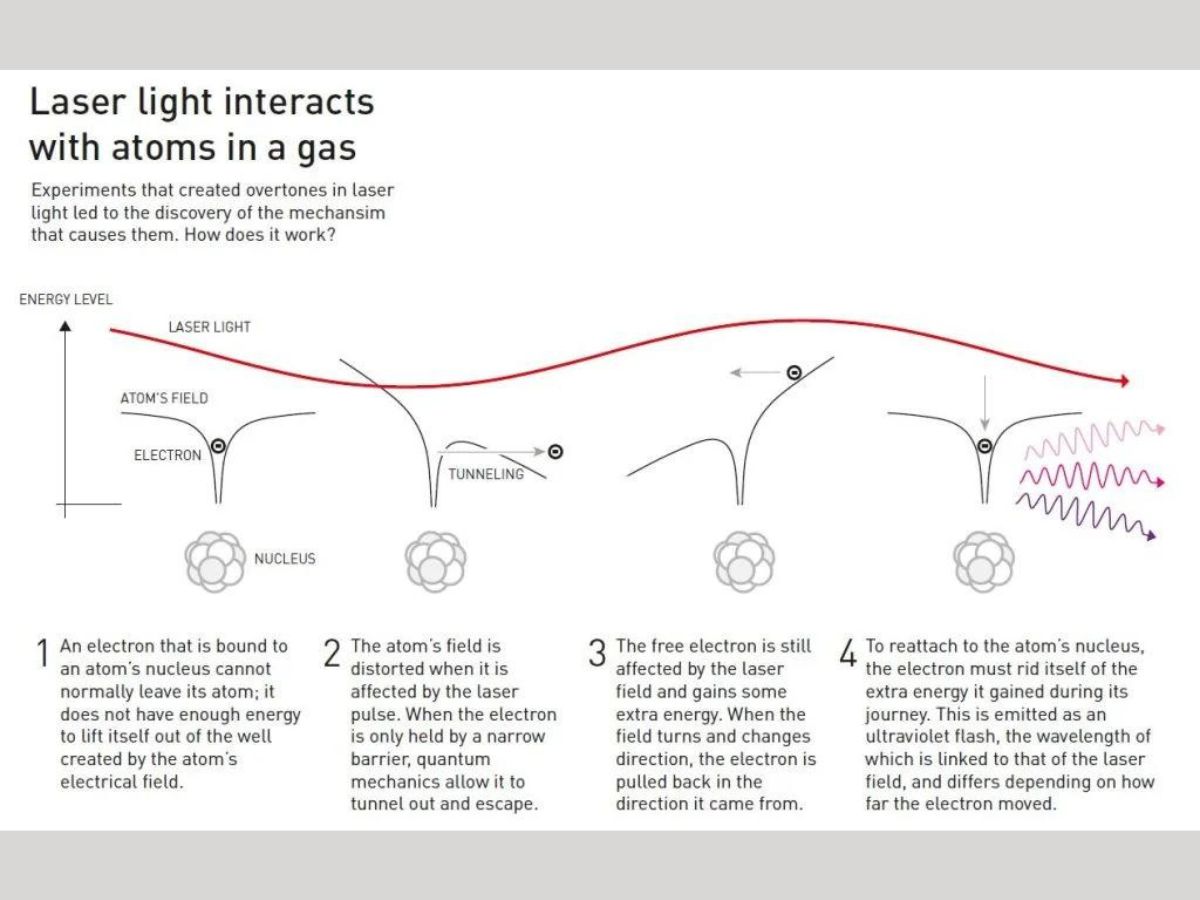
These pulses of light from the electrons are the overtones. The electrons emit ultraviolet flashes of light. The laser’s electric field, and the distance moved by the electron from the atom determine the wavelength of the ultraviolet pulse.
The wavelength of ultraviolet light is shorter than that of visible light. The wavelength of the overtone is directly proportional to the wavelength of the original laser pulse.
Depending on the atom with which the laser light interacts, different light waves with different wavelengths are produced.
Overtones can interact in different ways. When the peaks of overtones coincide, the light becomes more intense. When the peak of an overtone coincides with the trough of another overtone, the light becomes less intense.
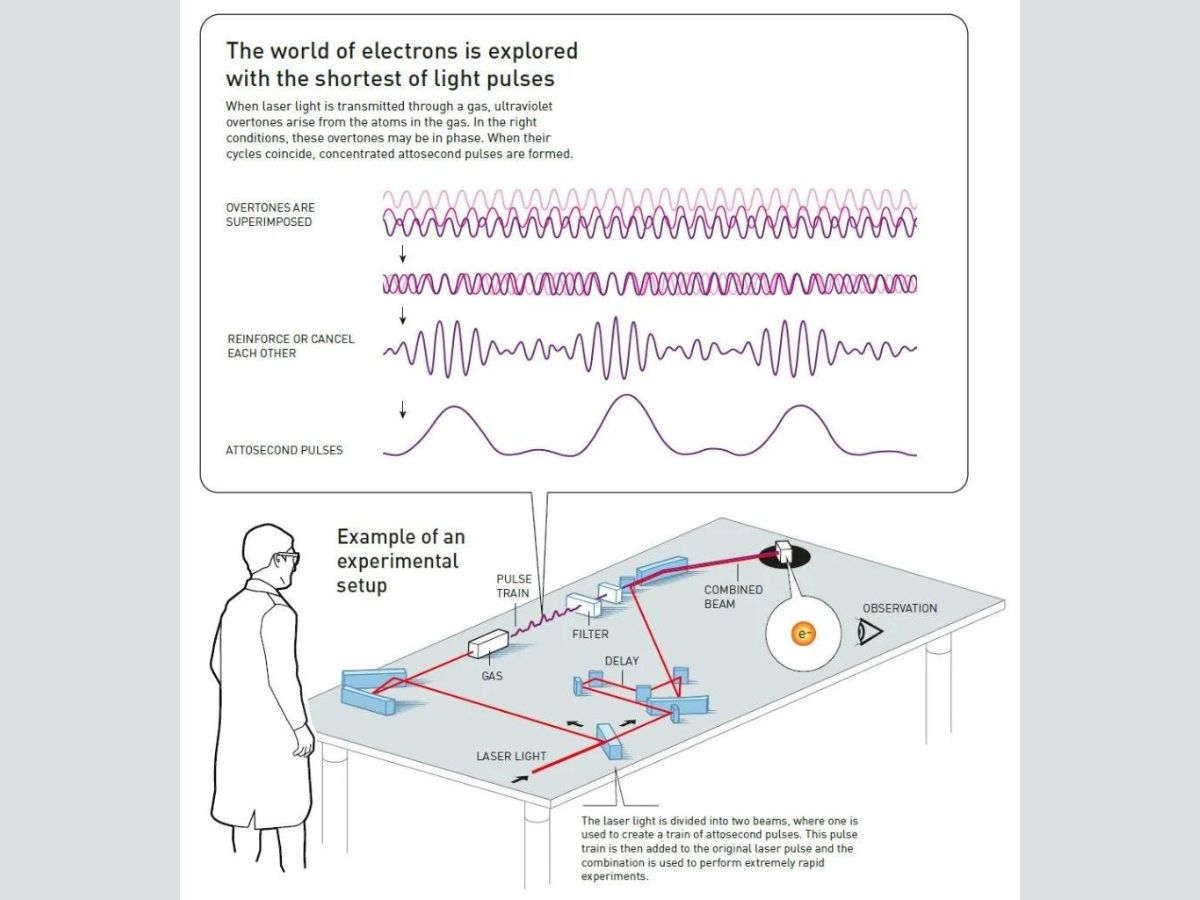
Therefore, in order to observe the movements of electrons, overtones should combine in such a way that a series of pulses of ultraviolet light are produced, where the wavelength of each pulse is a few hundred attoseconds. While the theory was understood in the 1990s, the pulses were identified and tested in reality in 2001.
Laser light transmitted through a gas results in the production of ultraviolet overtones due to energy released from electrons. The overtones are superimposed, and when the conditions are right, the overtones are in phase.
Some overtones reinforce each other, while others cancel each other. At the end, attosecond pulses are produced.
How the research progressed
L’Huillier, in 1987, transmitted an infrared laser beam through a noble gas, and showed that overtones can be produced. Infrared light was a perfect choice because it allowed the researchers to produce stronger and a larger number of overtones than what was possible using a laser with shorter wavelengths.
L’Huillier continued exploring this phenomenon during the 1990s.
In France, Agostini and his group produced and investigated a series of consecutive light pulses, similar to a train with carriages. This was done by dividing the original laser light into two beams, and using one to create a train of attosecond pulses. After that, the pulse train was combined with the other part of the original pulse. This allowed them to create pulses the duration of each of which was 250 attoseconds.
At the same time in Austria, Krausz and his research team worked on isolating a single pulse from a train of pulses. They successfully isolated a pulse that lasted 650 seconds.
All these breakthroughs in the world of attosecond physics allow scientists to study the movements of electrons. Attosecond pulses also allow scientists to measure the time taken by an electron to escape an atom’s field. This time allows scientists to determine how tightly the electron was bound to the atom’s nucleus.
In 2008, an electron was filmed for the first time. The movie showed how an electron moves over a light wave after it is pulled away from an atom. The study was published in the Physical Review Letters, and was co-directed by L’Huillier.
The practical applications of attosecond pulses include studying the internal processes of matter, exploring the world of particle physics, and in developing therapeutics. They are also extremely beneficial in the field of electronics.
Attosecond pulses can push molecules, resulting in the emission of a measurable signal that has a special structure. This special structure serves as a fingerprint that can reveal the type of molecule pushed by the pulses. Such techniques have immense potential in medical diagnostics.
[ad_2]
Source link